It is often rightly said “Nature is the Best Teacher”. The best treatment modalities in Medical and Dental field are often inspired by body natural healing mechanism.
Platelet derived growth factors are one such exemplary example. Today’s Regenerative Dentistry seems to be incomplete without inclusion of these Platelet Derived Growth Factors.
Amongst them Platelet Rich Fibrin (PRF) leads the front with a Mammoth amount of research evidence backing its effectiveness, spanning over decades.
History of the Platelet Rich Fibrin
Initial use of Platelet products was to control bleeding.
1970 – First Commercial Fibrin Sealant launched in Europe
1997- PRP (Platelet Rich Plasma) by Whitman (1st Generation)
1999 – PRGF (Platelet Rich Growth Factor) by Anitua
2001 – PRF (Platelet Rich Fibrin) by Dr. Choukroun (2nd Generation)
And lateron with refinement of protocol A-PRF (2013) & i-PRF (2015) (3rdGeneration)
PRF today not only has application in Dentistry but also in fields on Medicine & Surgery (Orthopedics, Cosmetics, ENT etc)
Contents
The 3 main contents of PRF –
- Cells
- Provisional Extracellular Matrix
- Bioactive Molecules
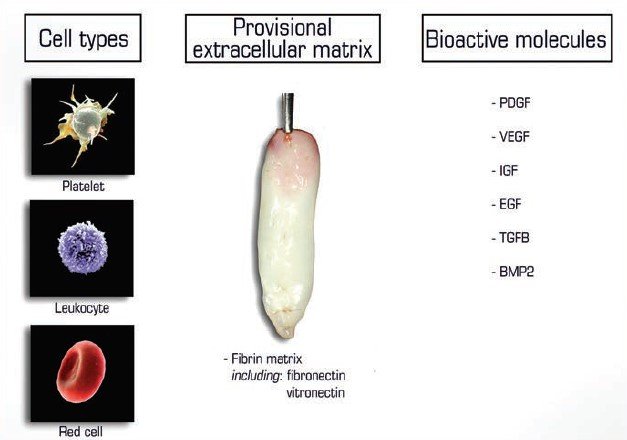
PRF serves various criteria’s for regeneration of tissues including 3D fibrin scaffold, includes autologous cells (platelets, leukocytes, macrophages, neutrophils etc) and serves as a natural reservoirs of growth factors.
METHOD OF PREPARATION
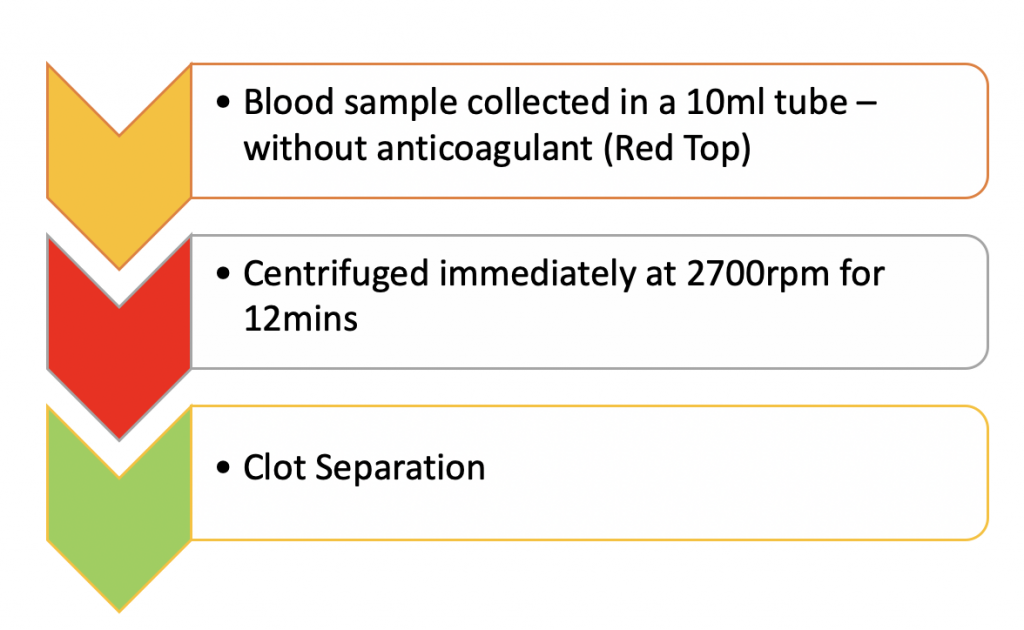
- PRF collection kit including 24 gauge butterfly needle and 9 ml blood collection tube.
Some centrifugues are available online like this one that could be use for the PRF protocol.
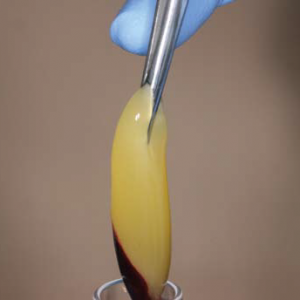
PRF Membranes
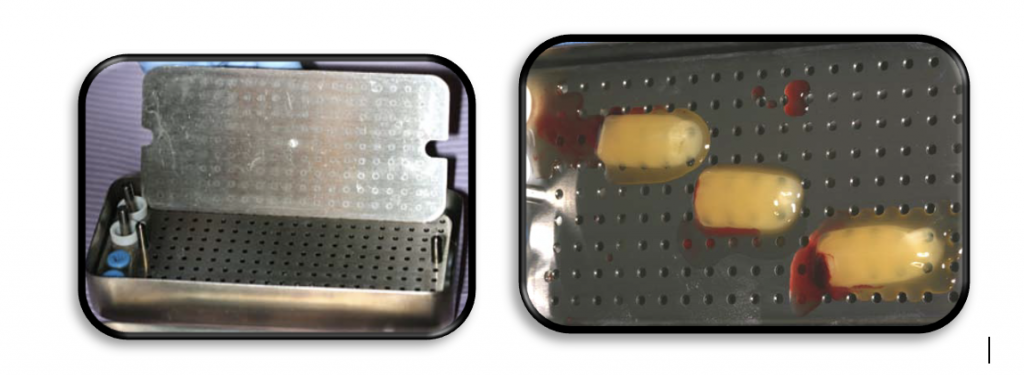
PRF membrane should be manipulates in special kits like this one.
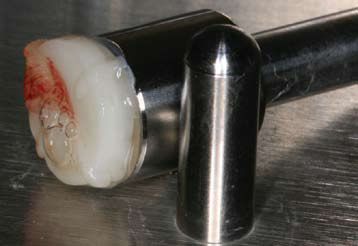
PRF Plugs
LOW SPEED CENTRIFUGATION CONCEPT
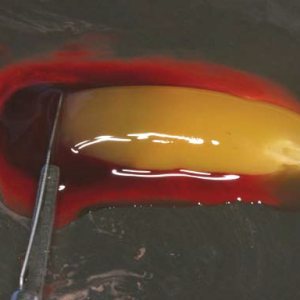
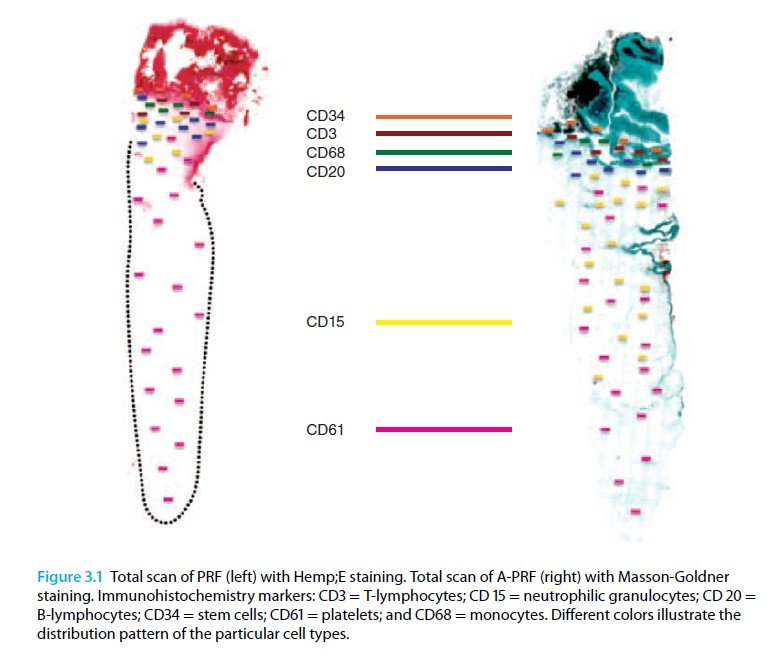
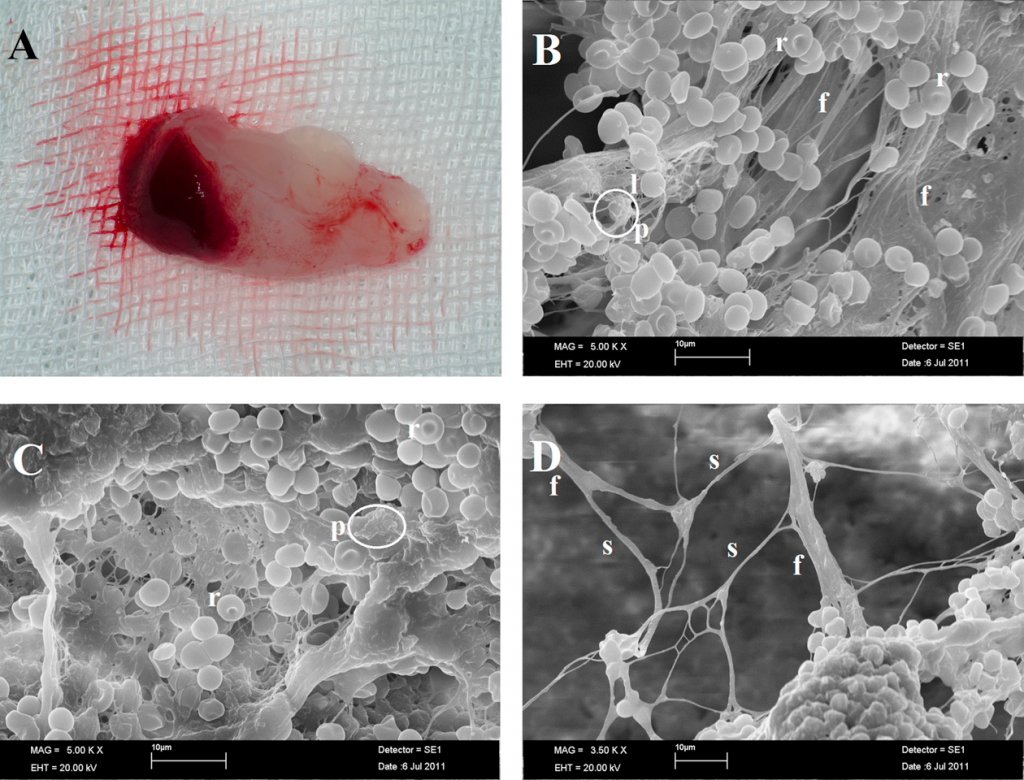
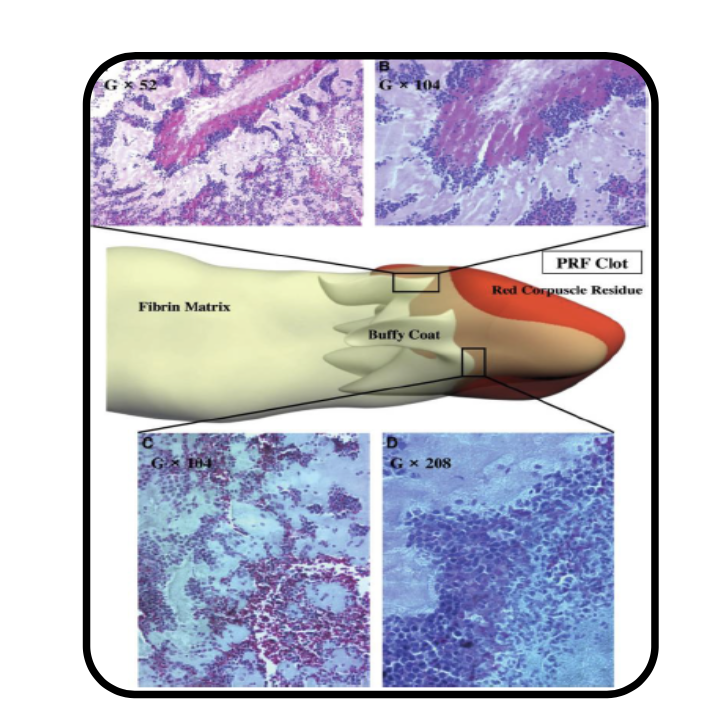
Application of high centrifugation force (708g) results in a dense fibrin network with minimum interfibrous space. Inflammatory cells distribution pattern is mostly seen in proximal part close to buffy coat, whereas platelet density decreases towards distal part.
When the centrifugation force is reduced (208g) [A-PRF protocol] a more porous structure with more interfibrous space is obtained. Cells have a more even distribution throughout the clot.
Reduction in centrifugation force not only resulted in more even distribution of cells but also enhanced number of included inflammatory cells.
A – PRF
- Introduced By Dr.Choukran in 2013
- Based on the low speed centrifugation concept
Method Of Preparation
10 mL of blood collected in a vacuum tube (Vacuum tube A-PRFTM , Biomedent. Cove West NSW, Australia) and centrifuged at 1300 rpm (208 g) for 8 min, followed by a 5 min resting step. Thereafter, the clot is extracted and the red blood cell fraction was carefully removed using surgical scissors.
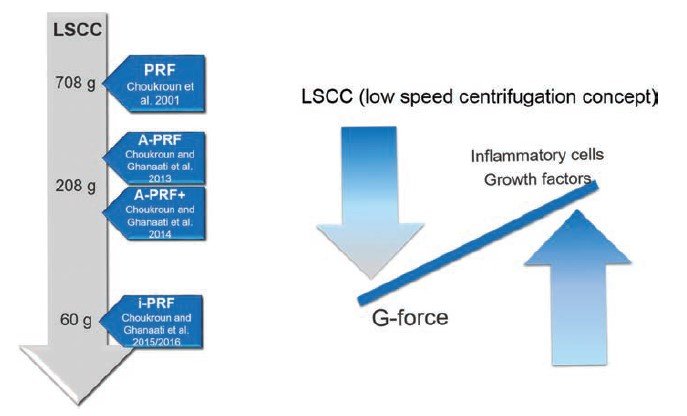
i-PRF
- Also called injectable PRF.
- Introduced in 2015 by Dr.Choukran.
Method Of Preparation
10 mL of blood collected in a vacuum tube (Vacuum tube i-PRFTM Choukroun, Biomedent. Cove West NSW, Australia) and centrifuged at 700 rpm (60 g) for 3 min. Following centrifugation, the orange supernatant (2 mL), representing the liquid i-PRF, is aspirated using a syringe fitted with a 21G needle.
Check this video on Desai Instagram how he prepares the PRF:
Steaky Bone
- Sticky bone is a stable fibrin bone graft
- It has its own body and is easy to handle and can be moulded into desired shape.
Method Of Preparation
i-PRF is poured over the bone graft granules and hydrated properly. Small bits of A-PRF membrane is also added to this mixture and mixed properly and is set aside for a few minutes.

T – PRF
- Based on the hypothesis – Titanium tubes – more effective in activating platelets than glass tubes
- 3rd Generation platelet concentrate
- Avoids any short or long tern effects of glass tube or silica
- Has an increased biocompatibility
- Other properties – similar to PRF
The fibrin of T-PRF seems to be more tightly woven & thicker than that of the classical PRF. The difference may be due to a better hemocompatibility of titanium compared to glass which could have potentially led to the formation of a more polymerized fibrin.
Hence its hypothesized that T-PRF may last a bit longer in the tissue.
In vivo study done by Mustafa Tunalı et al in 2013 showed that T-PRF could induce the formation of new bone with new connective tissue in a rabbit model of wound healing within 30 days of treatment.
Clinical Applications
Oral Applications:
- Implant Surface Bio-Modification
- Sinus Lifts
- Guided Bone Regeneration
- Periodontal Tissue Regeneration
- As a Bandage in wound healing etc
Extraoral Applications
- Tendon repair
- Burns
- Ulcers
- Facial plastic surgery
- Facial volumization
- Hair Loss treatment
- Depressed scar / Dermal augmentation etc
Properties
- Contains intimate assembly of cytokines, glycanic chains, structural glycoproteins enmeshed within a slowly ploymerized fibrin network – these are known to have established synergistic effects on healing
- Considered as an Immune node – known to stimulate defence mechanisms
- Significant inflammatory response at surgical site when treated with PRFM – Attributed to cytokines enmeshed in the fibrin matrix & released during remodelling of initial matrix
Role of Fibrin:
- Natural guide to angiogenesis
- Natural support to immunity
- Guides the coverage of injures tissue – affecting the metabolism of epithelial cells & fibroblasts
Advantages
- No bovine thrombin or anticoagulants – Safe
- Standard production protocol
- Slower release of growth factors – hence better healing
- Better organized fibrin matrix – more efficient direct stem cell migration & healing
- Acts as a supportive matrix for BMPs
- Helps in hemostasis
Advantages over other contemporary platelet products
- No Anticoagulant
- No Bovine Thrombin
- No Chemical Additives
- No Pipetting
- No Second spin
- No Expensive Consumables
Limitations
- Autologous – Hence limited volume – Small amount – can’t be used for general surgeries
- No anticoagulant used – Hence quick handling is required – Success depends on this
- Unfeasible for Tissue banks – as contains cytokines & growth factors – highly antigenic
Conclusion
A lot of platelet products have evolved with time.
In today’s times, regenerative procedures without inclusion of platelet products may seem incomplete.
With over two decades of use and more than 500 research papers, PRF speaks for itself.
It serves well as strong pillar in the triad of tissue Regeneration.
PRF can be considered as catalyst, aiding and speeding the healing process.
Its simplicity and versatility make it win over other contemporary platelet products.
With its ease of preparation to application it can be easily adapted into surgical work flow.
It can be considered as a modern-day boon in the field of regeneration.